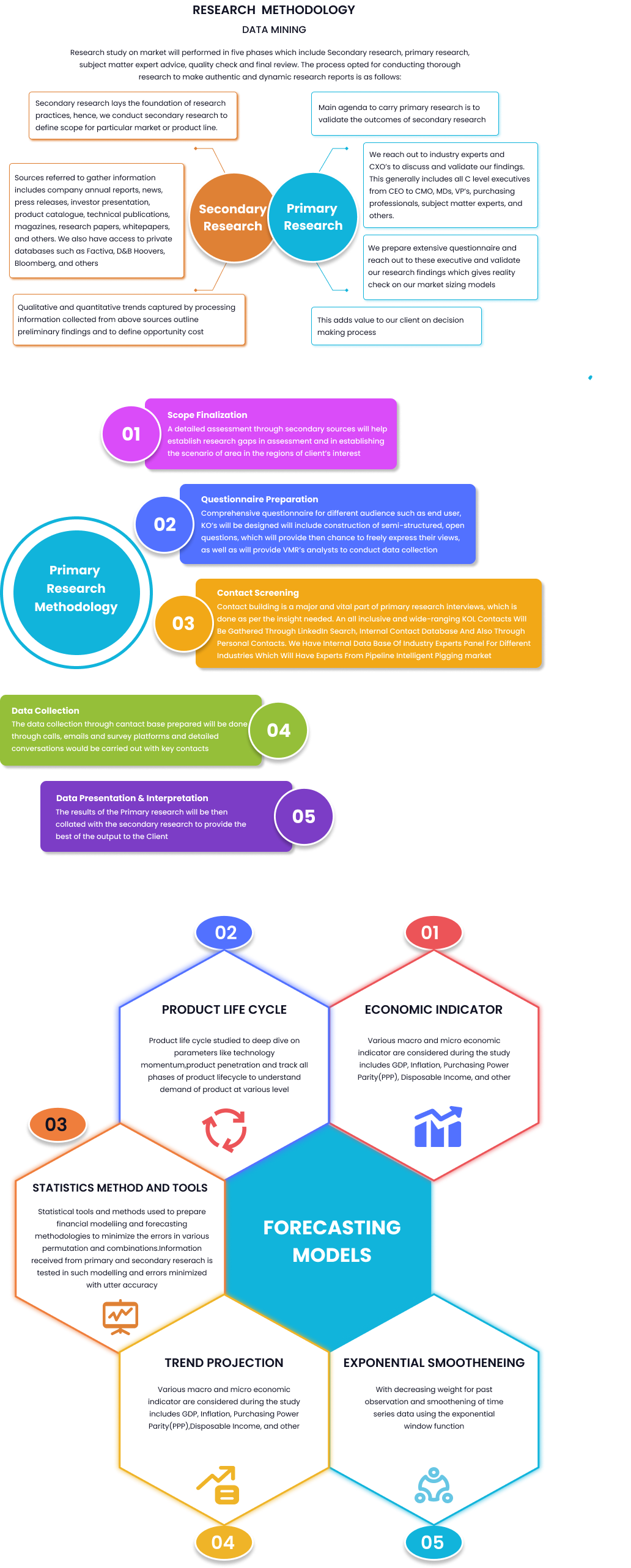
Global Competent Cells Market By Type (Chemically Competent Cells, Electro Competent Cells), By Application (Cloning, Protien Expression), By Region, And Segment Forecasts, 2023 to 2032
Report Id: 44151 | Published Date: May 2024 | No. of Pages: 10 | Base Year for Estimate: May 2024 | Format:
Market Overview:
Competent cells are bacterial cells that have been genetically altered to be able to take up foreign DNA molecules. If the cell walls of E. coli are changed, the cells tend to absorb the DNA. Calcium chloride and temperature shock therapy can make the cells competent. They play a crucial role in molecular biology research, particularly in recombinant DNA technology, gene cloning, and protein expression. Competent cells may also be utilised in therapeutic clonic research due to which these fields are anticipated to experience renewed attention which will lead to an increase in demand for these cells.
The Global Competent Cells market is valued at USD 1.92 billion in 2024 and is projected to reach USD 4.05 billion by 2029, growing at a CAGR of 9.81%. The cloning sector was not negatively affected by COVID-19 owing to corporations investing more money in research and development.
Premium Insights:
-The development of advanced competent cell technologies, such as chemically competent cells, electrocompetent cells, and high-efficiency transformation strains, is driving market growth by enhancing transformation efficiency and recombinant DNA manipulation capabilities.
- Increasing demand for customized competent cell solutions tailored to specific research requirements, including cloning, mutagenesis, and protein expression, is fueling market expansion and innovation in the healthcare sector.
- Strategic collaborations between competent cell manufacturers and academic research institutions, as well as biopharmaceutical companies, are facilitating technology transfer, product development, and market penetration, contributing to market competitiveness and growth.
Global Competent Cells Market Dynamics :
Drivers: Molecular biology techniques, technological advancements and other factors
-The increasing adoption of molecular biology techniques, such as gene cloning, CRISPR-Cas9 genome editing, and recombinant protein expression, is driving the demand for competent cells in academic research and biopharmaceutical industries.
- Technological advancements, such as improved transformation efficiencies, enhanced stability of recombinant DNA constructs, and the development of novel gene editing tools, are expanding the utility and applications of competent cells in healthcare research and development.
-Increasing adoption of molecular cloned products, and recombinant proteins along with several government initiatives and grants that are available for life science research are also some of the key factors driving industry growth.
Restraints: Expensive Competent Cell Products and stringent regulations.
-The high cost of competent cell products, particularly specialized strains and custom formulations, can act as a barrier to market entry for small and medium-sized research laboratories and academic institutions, limiting market growth potential.
-Stringent regulatory requirements governing the use of genetically modified organisms (GMOs) and recombinant DNA technologies may pose challenges for market players in terms of compliance, product registration, and market access, particularly in regions with strict biosafety regulations.
Opportunities: Focus on personalised medicine, favourable governmental initiatives, advancements in recombinant DNA technology, the emergence of gene therapy
- The increasing focus on personalised medicine, precision diagnostics, and synthetic biology applications presents significant opportunities for market expansion and product diversification in the competent cells market.
- Favourable initiatives by the government across the world to promote research, especially by emerging countries have led to an increase in FDA approval for gene therapy products and advancement in research as well as other developmental activities
- Recombinant DNA technology- one of the most commonly used techniques used for introducing a specific gene or a healthy gene into a vector has experienced recent advancements in recombinant DNA synthesis that have enabled the effective production of recombinant proteins at industrial sizes with higher yields and lower production costs, enabling development new treatments, diagnostic reagents and vaccines
- The emergence of gene therapy, cell therapy, and regenerative medicine as promising treatment modalities is driving the demand for competent cells for gene editing, gene delivery, and cell line engineering applications.
Market By Competent Cells Type Insights:
Based on the type, the market is segmented into Electro Competent Cells and Chemically Competent Cells. The Chemically Component Cells Segment is predicted to hold the largest market share. They are expected to present high growth at a CAGR of 15.5% in 2023 and 2024, The Electro Competent Cells Segment is also expected to grow as they are suitable for many molecular biology techniques like the creation of DNA libraries.
Market By End Use Insights:
Based on End Users, academic and research institutes represent the largest end-user segment, driven by the extensive use of competent cells in molecular biology, genetics, and biotechnology research although Biotechnology and pharmaceutical companies are major consumers of competent cells for recombinant protein production, drug discovery, and biomanufacturing applications.
Market by Region Insights:
Based on regional coverage, the Global Competent Cells Market can be segmented into North America, Asia-Pacific, and Europe. The largest share in the market was dominated by The USA in 2021 and will continue to do so due to the growing number of R and D activities in the region.
Competitive Scenario:
Key players operating in the Global Competent Cells Market include Thermo Fisher Scientific, Inc., Merck KGaA, Promega Corporation, Takara Bio, Inc., New England Biolabs, Inc., Bio-Rad Laboratories, Inc., Transgen Biotech, Inc., Agilent Technologies, Inc., Illumina, Inc., Zymo Research, Qiagen N.V., Genscript Biotech Corporation, and Origene Technologies, Inc. and others.
Scope of Work- Global Competent Cells Market
Key Market Developments:
September 2023- Merck KGaA launched its next-generation competent cells, including the SuperNova competent cells and the PrimeStar competent cells, offering enhanced transformation efficiency and cloning performance for a wide range of applications.
November 2024- Thermo Fisher Scientific Inc. announced the acquisition of a leading provider of electrocompetent cells and molecular biology reagents, strengthening its position in the global competent cells market and expanding its product offerings.
June 2019- The Guangdong Provincial Government and the Chinese Society of Bioengineering collaborated to support the China Bio-Industry Conference. The Guangzhou Nanshan Science and Technology Innovation Fund, a public-private partnership, was announced during the event and will provide RMB 200 million in funding to biotech companies that are willing to conduct research in the region.
Frequently Asked Questions (FAQs)
What are the key applications of competent cells in healthcare?
Ans. Competent cells are used in various healthcare applications such as recombinant protein production, gene cloning, genome editing, drug discovery, and vaccine development.
What are the challenges associated with the use of competent cells in healthcare research?
Ans. Challenges include high costs of competent cells and reagents, variability in transformation efficiency, ethical and regulatory concerns surrounding GMOs, and the need for specialized expertise and equipment for complex cloning projects and genome editing applications.
What are the key trends shaping the competent cells industry?
Ans. The increased prevalence of genetic disorders and investment in research and development are some of the key trends in the market.
Which countries are the key producers of competent cells?
Ans. The USA, China, and Germany are the key producers of competent cells

Speak with an analyst to get exclusive insights tailored to your needs
.png)