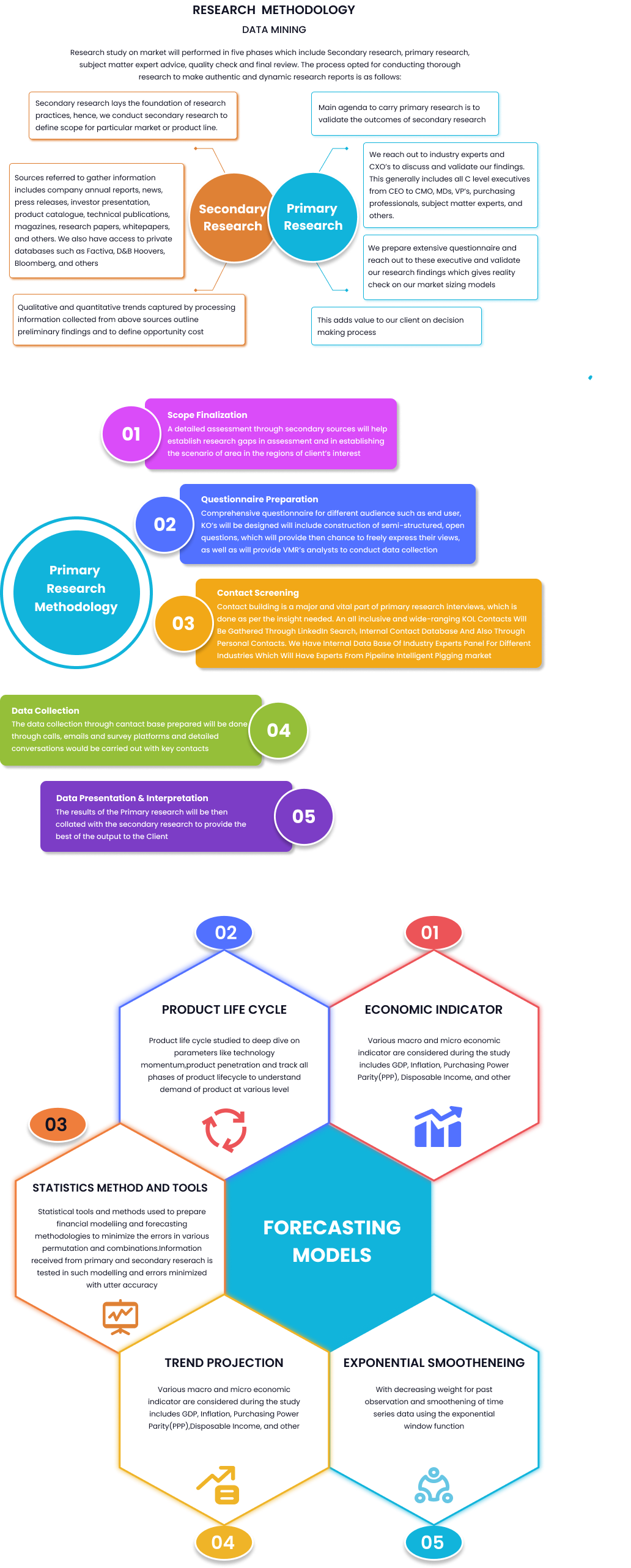
Global Lentiviral Vectors Market By Type (First Generation, Second Generation, Third Generation), By Application (Hospitals, Clinics, Research Institutes), By Region, And Segment Forecasts, 2023 to 20...
Report Id: 44152 | Published Date: May 2024 | No. of Pages: 10 | Base Year for Estimate: May 2024 | Format:
Market Overview:
The global lentiviral vectors market in the healthcare system is experiencing robust growth, driven by the increasing adoption of lentiviral vectors in gene therapy, cell therapy, vaccine development, and research applications. Lentiviral vectors are versatile gene delivery tools derived from lentiviruses, capable of efficiently providing genetic material to a wide range of dividing and non-dividing cells, making them invaluable tools for gene transfer and expression.
The Global Lentiviral Market is valued at USD 11.2 million in 2024 and is expected to reach USD 25.34 million by 2030, at a CAGR of 12%during the forecast period. Lentiviral vectors have become powerful vectors for vaccination because of their high efficacy in transferring dendritic cells, including CD8+ T cells, long-lasting antibody-mediated immunity, and excellent protection in a range of pre-clinical animal models of infection and malignancies. Their demand has increased in recent years because of researchers using them for the development of COVID-19 vaccines.
Premium Insights:
-Lentiviral vectors offer several advantages over other gene delivery systems, including stable integration into the host genome, high transduction efficiency, long-term gene expression, and low immunogenicity, making them ideal candidates for gene therapy applications. They are also frequently used in fundamental biology research due to their unique features.
-The growing prevalence of genetic disorders, cancers, and infectious diseases, coupled with advancements in gene editing technologies such as CRISPR-Cas9, is driving the demand for lentiviral vectors for precision medicine and personalized therapies.
-The expanding applications of lentiviral vectors in cell-based therapies, including chimeric antigen receptor (CAR) T-cell therapy, stem cell therapy, and immunotherapy, are driving market growth by enabling the development of novel treatment modalities for a wide range of diseases.
Global Lentiviral Vectors Market Dynamics:
Drivers: Demand for gene therapy, advancements in gene editing technologies, varied applications and others
-The growing demand for gene therapy for the treatment of monogenic disorders, rare diseases, and cancers is driving the adoption of lentiviral vectors as efficient gene delivery vehicles for delivering therapeutic genes into target cells and tissues. For example, on May 14, 2020, pre-clinical data for AVR-RD-03 for Pompe disease were presented by AVROBIO, Inc., a clinical-stage gene therapy company. The study proved that lentiviral gene therapy was effective in treating Pompe disease symptoms that affect the central nervous system (CNS) and muscles
- The continuous advancements in cell and gene editing technologies, such as CRISPR-Cas9, TALENs, and zinc finger nucleases, are fueling the demand for lentiviral vectors for delivering gene editing tools and aiding in precise genome engineering.
- The expanding applications of lentiviral vectors in cell therapy, including CAR T-cell therapy, stem cell therapy, and adoptive cell therapy, are driving market growth by enabling the development of next-generation cell-based therapeutics with enhanced efficacy and safety profiles.
- The market for lentiviral vectors is anticipated to expand as a result of major players concentrating on different inorganic growth tactics like partnerships and agreements. For example, in May 2017, bluebird bio, Inc. and Novartis Pharma AG signed a licence agreement. To develop and market oncology therapies, Novartis will nonexclusively license certain Bluebird patent rights of lentiviral vector technology.
Restraints: Manufacturing complexities, limited cargo capacity among others
-The complexity and cost of manufacturing lentiviral vectors at a large scale pose challenges to commercialization and widespread adoption, particularly for cell and gene therapy products requiring high vector titers and purity levels for clinical applications.
- The limited cargo capacity of lentiviral vectors for packaging large transgenes or multiple gene payloads may restrict their utility for certain gene therapy applications requiring delivery of large genetic constructs or complex gene expression systems.
- Safety concerns related to the risk of insertional mutagenesis, oncogenesis, and immune responses associated with lentiviral vector-mediated gene transfer pose challenges to market growth and regulatory approval, necessitating stringent safety assessments and regulatory oversight.
Opportunities: increased demand for cancer treatments, the emergence of next-gen lentiviral sector platforms.
- There is a growing demand for lentiviral vectors in T cell engineering for cancer treatment. Gene-modified cells have already benefited from the use of lentiviral vectors in the development of T-cell therapies in particular. Chimeric antigen receptors (CARs) or cloned T-cell receptors are also delivered to mature T cells via these vectors to stimulate anti-cancer immunity. Alongside CAR T-cell therapy, lentiviral vectors have undergone changes and evolution, and they are now showing all of their potential as an adjuvant therapy and a potent, supplementary instrument in the treatment of a wider variety of cancers.
-The emergence of next-generation lentiviral vector platforms, such as self-inactivating (SIN) vectors, third-generation vectors, and engineered capsids with improved transduction efficiency and safety profiles, presents significant opportunities for market players to develop innovative lentiviral vector technologies tailored for specific therapeutic and research applications.
Market by Lentiviral Vectors Type Insights :
Based on Type the Global Lentiviral Market is segmented into 1st generation, 2nd generation, 3rd generation. The 3rd generation lentiviral vectors dominate the market share as they incorporate additional safety features, such as the deletion of viral accessory genes and the inclusion of regulatory elements to enhance transgene expression and vector stability.
Market By End Use Insights
Based on End Use, Biopharmaceutical companies represent the largest end-user segment, driven by their extensive investments in gene therapy, cell therapy, and vaccine development pipelines leveraging lentiviral vector technologies. Academic and research institutes play a crucial role in advancing lentiviral vector research, technology development, and preclinical studies, contributing to the growth of the market through research collaborations and technology transfer initiatives.
Market by Region Insights:
Based on regional coverage, the global lentiviral vectors market is segmented into North America, Asia-Pacific, Europe, and Middle East Africa (MEA). North America dominates the global lentiviral vectors market, driven by the presence of key biotechnology hubs, academic research centres, and pharmaceutical companies driving innovation and market growth. However, due to the high concentration of significant manufacturers in the United States and the rising incidence of cancers including bladder, oesophagal, liver, and pancreatic cancer, Asia-Pacific is predicted to grow at the fastest rate between 2023 and 2030. In addition, technological development and increased foreign investment.
Competitive Scenario :
Key players, operating in the Global Lentiviral Vectors Market are Cobra Biologics Limited, Sirion-Biotech GmbH, Merck KGaA, FinVector Oy, Oxford Biomedica, OriGene Technologies, Inc., Sino Biological Inc., Cell Biolabs, Inc., Thermo Fisher Scientific Inc. and others.
Scope of Work- Global Lentiviral Vectors Market
Key Market Developments :
December 2023- Thermo Fisher Scientific Inc. announced the launch of its next-generation lentiviral vectors for enhanced gene delivery and expression in gene therapy and cell therapy applications, offering improved safety and efficacy profiles.
August 2024 - Merck KGaA announced the expansion of its lentiviral vector manufacturing facility in the United States to meet the growing demand for lentiviral vectors for cell and gene therapy applications, strengthening its position in the global market.
Frequently Asked Questions (FAQs)
What are the key applications of lentiviral vectors in healthcare?
Ans. Lentiviral vectors are used in gene therapy, cell therapy, vaccine development, and research applications for treating genetic disorders, cancers, infectious diseases, and regenerative medicine
What factors are driving the growth of the global lentiviral vectors market?
Ans. The growth of the global lentiviral vectors market is primarily driven by the increasing demand for gene therapy, advancements in cell and gene editing technologies, expanding applications in cell therapy and immunotherapy, and the emergence of next-generation lentiviral vector platforms with improved safety and efficacy profiles.
What regions are witnessing significant growth in the lentiviral vectors market?
Ans. North America, Europe, and the Asia Pacific are witnessing significant growth in the lentiviral vectors market.
What are the emerging trends and opportunities in the lentiviral vectors market?
Ans. Emerging trends include the development of next-generation lentiviral vector platforms, advancements in cell and gene editing technologies, expanding applications in cell-based therapies, and increasing investments in research and development.

Speak with an analyst to get exclusive insights tailored to your needs
.png)